The Ideal Gas Assumption and Boyle’s Law: What Remains Constant In Boyles Law
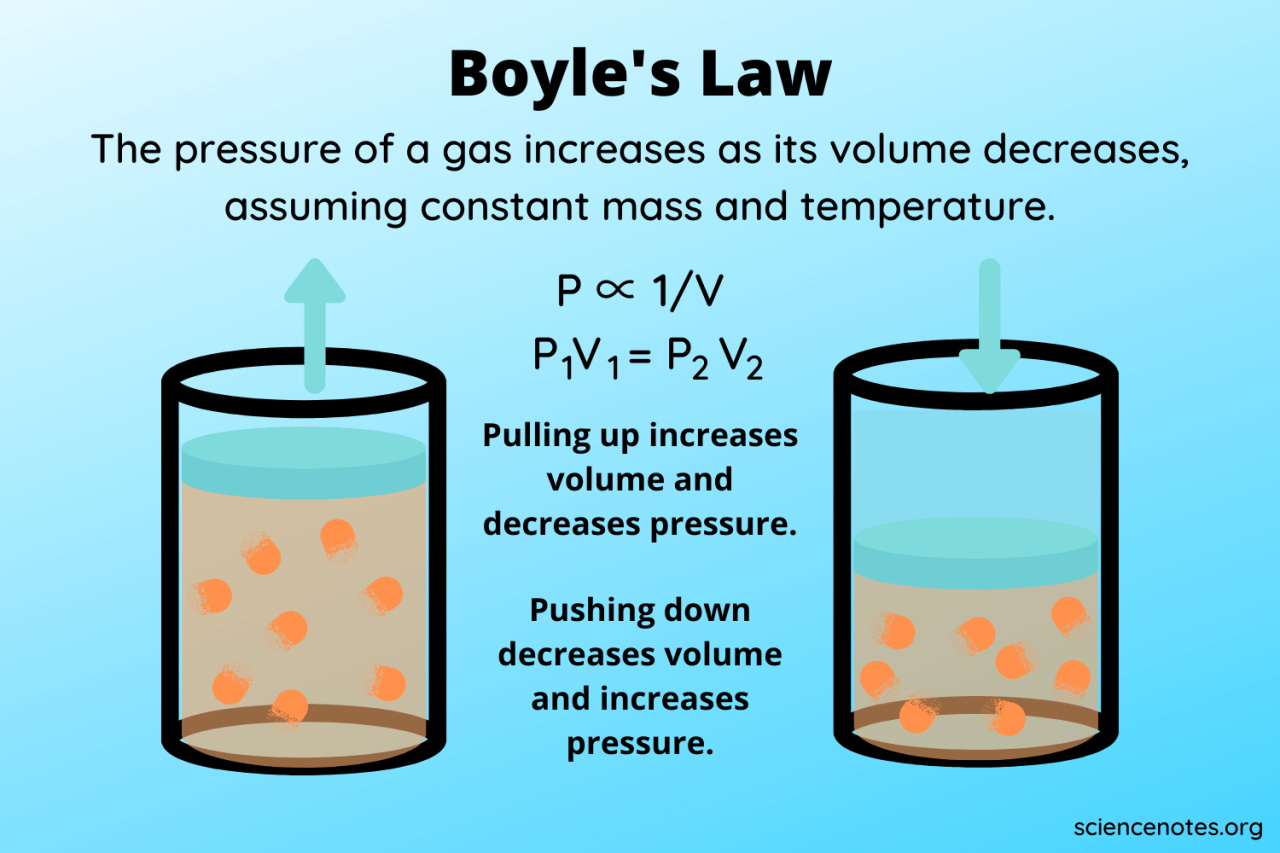
Boyle’s Law, stating that the pressure and volume of a gas are inversely proportional at constant temperature, is a fundamental concept in chemistry. However, its application relies on the ideal gas assumption, a simplification of real gas behavior. Understanding the limitations of this assumption is crucial for accurately predicting gas behavior in various real-world scenarios.
Assumptions of Ideal Gas Behavior and Their Implications for Boyle’s Law
The ideal gas law assumes that gas particles have negligible volume and do not interact with each other (no intermolecular forces). These assumptions simplify calculations significantly, but real gases deviate from this ideal behavior, particularly at high pressures and low temperatures. These deviations impact the accuracy of Boyle’s Law predictions. At high pressures, the volume of the gas particles themselves becomes significant compared to the total volume, causing the observed volume to be larger than predicted by the ideal gas law. At low temperatures, intermolecular forces become more prominent, leading to increased attraction between gas particles, causing the observed pressure to be lower than predicted.
Gases Deviating Significantly from Ideal Behavior
Several gases deviate significantly from ideal gas behavior. For instance, polar molecules like ammonia (NH₃) and water vapor (H₂O) exhibit strong intermolecular forces due to dipole-dipole interactions and hydrogen bonding, respectively. These strong attractions cause substantial deviations from Boyle’s Law, especially at lower temperatures and higher pressures. Similarly, gases with large molecules, such as butane (C₄H₁₀), exhibit noticeable deviations due to the significant volume occupied by the molecules themselves. Noble gases, while generally closer to ideal behavior, still show deviations at extremely high pressures.
The Role of Intermolecular Forces in Boyle’s Law, What remains constant in boyles law
Intermolecular forces play a critical role in determining how well a real gas adheres to Boyle’s Law. Attractive forces between gas molecules cause them to be closer together than predicted by the ideal gas model, resulting in a lower pressure than expected at a given volume. Repulsive forces, dominant at high pressures, cause the gas molecules to occupy more space than predicted, leading to a higher volume than expected at a given pressure. The strength of these forces varies depending on the type of gas and its temperature.
Comparison of Ideal and Real Gas Behavior under Boyle’s Law Conditions
The following table compares the behavior of an ideal gas and a real gas (e.g., carbon dioxide) under conditions relevant to Boyle’s Law. Note that the deviation from ideal behavior is a qualitative assessment and can be quantified using various methods like the compressibility factor (Z).
| Gas Type | Pressure (atm) | Volume (L) | Temperature (K) | Deviation from Ideal Behavior |
|---|---|---|---|---|
| Ideal Gas | 1 | 10 | 298 | None |
| CO₂ (Real Gas) | 1 | 10.1 | 298 | Slight positive deviation (due to intermolecular forces) |
| Ideal Gas | 10 | 1 | 298 | None |
| CO₂ (Real Gas) | 10 | 0.8 | 298 | Significant positive deviation (increased volume of CO2 molecules is significant) |

Tim Redaksi