Introduction to Charles’s Law: Which Variable Is Constant In Charles Law
Charles’s Law describes the relationship between the volume and temperature of a gas when the pressure and the amount of gas are held constant. In simpler terms, it states that as the temperature of a gas increases, its volume also increases proportionally, and vice versa, assuming the pressure remains unchanged. This direct relationship is fundamental to understanding the behavior of gases under varying temperature conditions.
Charles’s Law explains why a balloon inflates when heated and deflates when cooled. The heat energy causes the gas particles within the balloon to move faster and collide more frequently with the balloon’s inner surface, increasing the pressure. To maintain a constant pressure (as the balloon is not sealed tightly), the balloon expands, increasing its volume. Conversely, cooling the balloon reduces the kinetic energy of the gas particles, leading to slower movement and fewer collisions, resulting in a decrease in volume.
Mathematical Expression of Charles’s Law
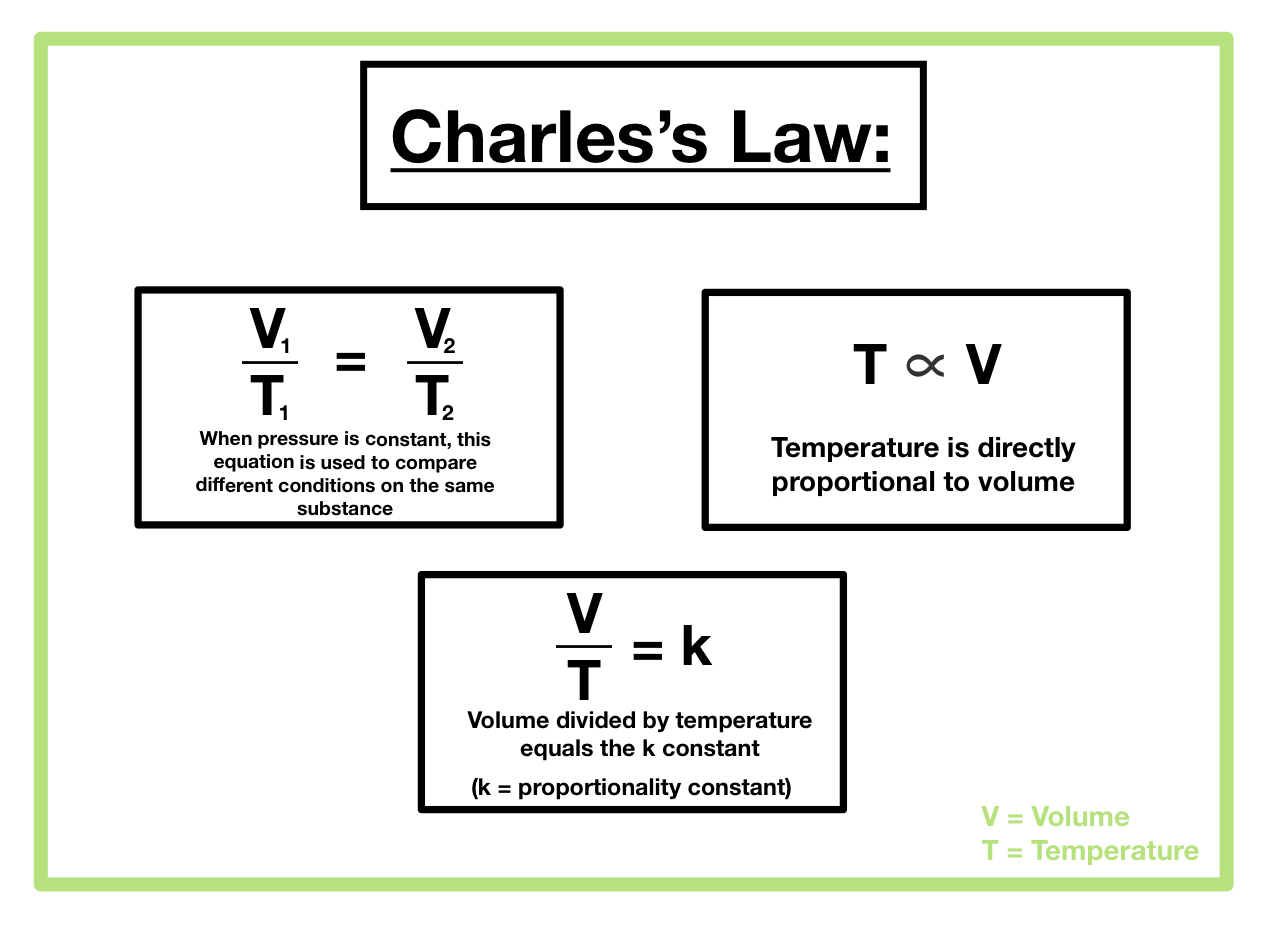
Charles’s Law can be expressed mathematically as:
V₁/T₁ = V₂/T₂
where:
* V₁ represents the initial volume of the gas
* T₁ represents the initial temperature of the gas (in Kelvin)
* V₂ represents the final volume of the gas
* T₂ represents the final temperature of the gas (in Kelvin)
It is crucial to note that temperature must always be expressed in Kelvin (K) for Charles’s Law calculations. Using Celsius or Fahrenheit will lead to inaccurate results. The Kelvin scale is an absolute temperature scale, meaning it starts at absolute zero (0 K), where theoretically all molecular motion ceases.
Real-World Example of Charles’s Law, Which variable is constant in charles law
A hot air balloon provides a clear demonstration of Charles’s Law. The burner heats the air inside the balloon, causing the air to expand. This expansion increases the volume of the air within the balloon, making it less dense than the surrounding cooler air. The buoyant force from the less dense, heated air within the balloon is then greater than the weight of the balloon and its payload, causing the balloon to rise. As the air inside the balloon cools, the volume decreases, and the balloon descends. This process relies directly on the relationship between temperature and volume as described by Charles’s Law.

Tim Redaksi