Introduction to Boyle’s Law
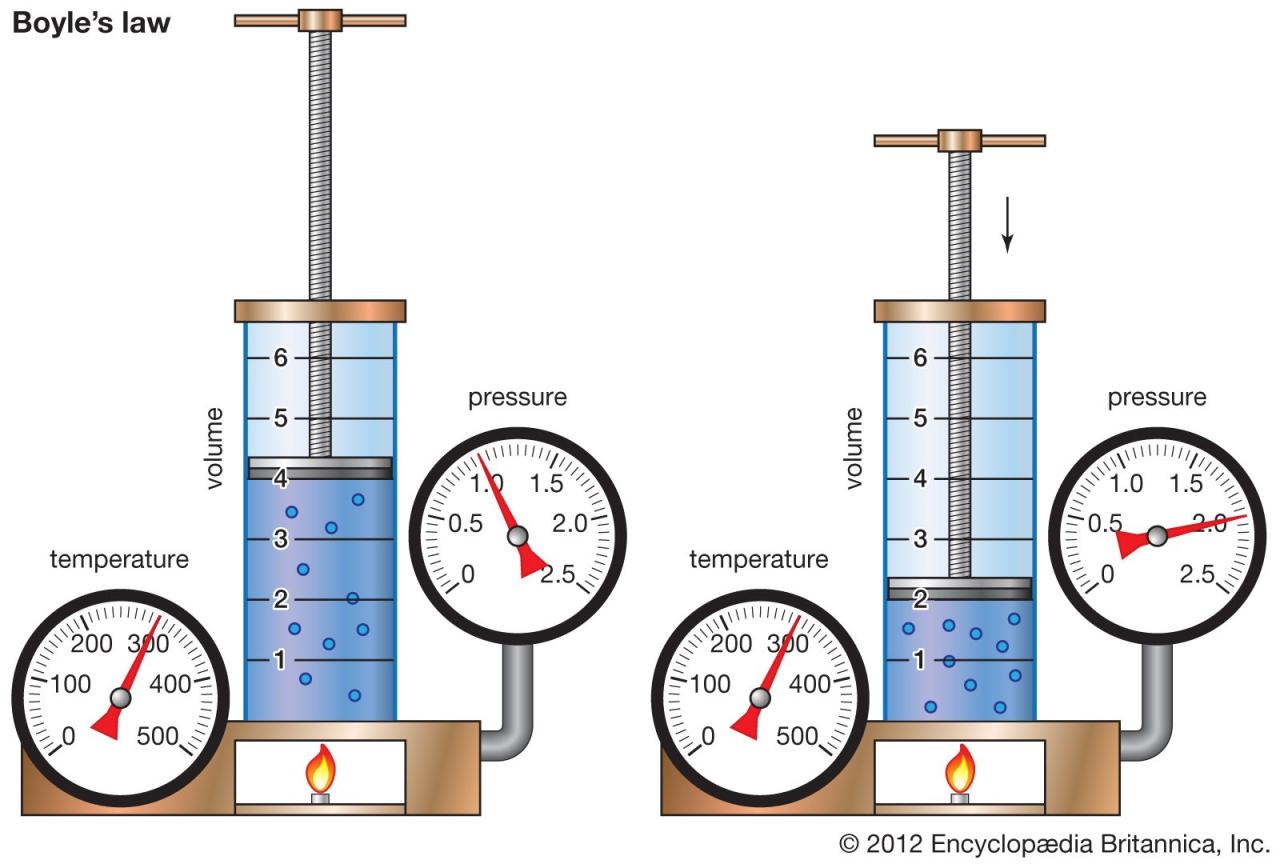
Boyle’s Law is a fundamental principle in physics that describes the inverse relationship between the pressure and volume of a gas, provided the temperature and the amount of gas remain constant. In simpler terms, if you squeeze a gas into a smaller space (decreasing its volume), its pressure will increase. Conversely, if you allow the gas to expand (increasing its volume), its pressure will decrease. This relationship is crucial in understanding how gases behave in various situations, from inflating balloons to the operation of internal combustion engines.
Boyle’s Law, while bearing his name, wasn’t solely his discovery. Early experiments hinting at this relationship were conducted by several scientists, but Robert Boyle, in 1662, meticulously documented his experiments and provided a comprehensive mathematical description of the inverse relationship between pressure and volume. He used a J-shaped tube, trapping a fixed amount of air in one arm and varying the pressure by adding mercury to the other. His careful observations and quantitative analysis established the law’s validity and significantly advanced the understanding of gases, laying the groundwork for future developments in thermodynamics and chemistry. The significance of Boyle’s work lies not only in the discovery itself but also in its rigorous experimental methodology, which served as a model for future scientific investigations.
A Definition of Boyle’s Law
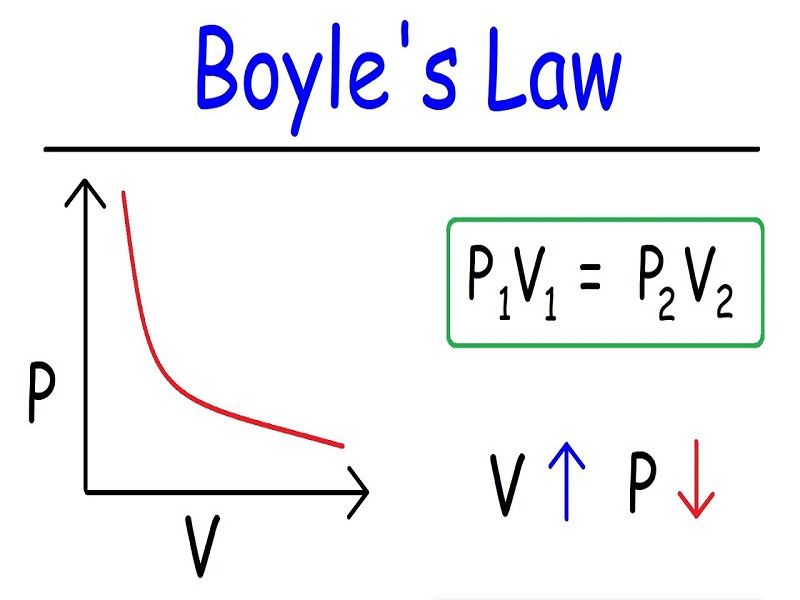
Boyle’s Law states that for a fixed amount of gas at a constant temperature, the pressure (P) and volume (V) are inversely proportional. This relationship can be expressed mathematically as:
P1V1 = P2V2
where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume. This equation highlights the inverse proportionality: if pressure increases, volume decreases proportionally, and vice versa, while the product of pressure and volume remains constant. This simple yet powerful law finds applications in diverse fields, from designing scuba diving equipment to understanding the behavior of gases in the atmosphere.
Applications of Boyle’s Law: What Is The Relationship Between The Variables In Boyles Law
Boyle’s Law, describing the inverse relationship between pressure and volume of a gas at constant temperature, has far-reaching implications in numerous scientific fields and everyday scenarios. Its principles are fundamental to understanding and manipulating gaseous systems, impacting technologies and natural processes alike. This section explores several key applications, highlighting the practical relevance of this foundational gas law.
What is the relationship between the variables in boyles law – The impact of Boyle’s Law is pervasive, influencing everything from the functioning of our lungs to the design of sophisticated engineering systems. Understanding how pressure and volume changes affect gases is crucial in various fields, allowing for precise control and prediction of gaseous behavior in a wide range of applications.
Scuba Diving and Boyle’s Law
Boyle’s Law is critically important for scuba diving safety. As divers descend, the pressure of the surrounding water increases. According to Boyle’s Law, this increased pressure causes the volume of air in the diver’s lungs and other air spaces (such as the sinuses and middle ear) to decrease. Conversely, as divers ascend, the pressure decreases, and the volume of air increases. Failure to account for these volume changes can lead to serious injuries, such as lung overexpansion (a potentially fatal condition) during ascent or ear barotrauma during descent. Divers must learn to equalize the pressure in their air spaces by adding air as they descend and releasing air as they ascend. This careful management of air volume directly reflects the practical application of Boyle’s Law.
Respiratory System and Boyle’s Law
Our respiratory system is a prime example of Boyle’s Law in action. Breathing involves the rhythmic expansion and contraction of the lungs. When the diaphragm and intercostal muscles contract, the volume of the chest cavity increases. This increase in volume leads to a decrease in pressure within the lungs, causing air to rush in (inhalation). Conversely, when the diaphragm and intercostal muscles relax, the volume of the chest cavity decreases, increasing the pressure within the lungs and forcing air out (exhalation). This cyclical process of pressure and volume changes, governed by Boyle’s Law, is essential for the continuous exchange of oxygen and carbon dioxide in our bodies.
Pneumatic Systems and Boyle’s Law
Pneumatic systems, which utilize compressed air to power machinery and tools, heavily rely on the principles of Boyle’s Law. These systems use compressed air stored in tanks at high pressure. When the air is released through valves and actuators, it expands, causing a decrease in pressure and an increase in volume, which performs the desired work. Examples include pneumatic drills, brakes in vehicles, and industrial robots. The design and operation of these systems require a thorough understanding of how pressure and volume changes affect the performance and efficiency of the compressed air.
Everyday Applications of Boyle’s Law, What is the relationship between the variables in boyles law
Many everyday items and processes implicitly use Boyle’s Law. For example, aerosol cans utilize compressed gas to propel their contents. The pressure inside the can forces the liquid or other substance out when the valve is opened. Similarly, syringes function based on the principle of Boyle’s Law: pulling back on the plunger increases the volume of the syringe, reducing the pressure inside and drawing fluid in. The opposite happens when the plunger is pushed, increasing the pressure and forcing the fluid out. These are commonplace examples demonstrating the practical and often unnoticed influence of Boyle’s Law in our daily lives.

Tim Redaksi